Intranasal vaccine Phase 1 trials of Bharat Biotech commences
The Hyderabad based vaccine manufacturer, Bharat Biotech has begun the first phase of clinical trials of the intranasal Covid-19 vaccine which is being developed in collaboration with the Washington University School of Medicine.
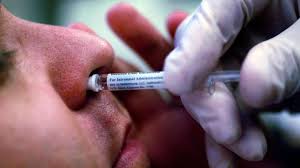
The Phase 1 human clinical trials of Bharat Biotech’s intranasal Covid-19 vaccine, codenamed BBV154, have commenced at some of the selected sites that include Hyderabad and Nagpur.
The trials of the chimpanzee adenovirus vectored vaccine candidate, being developed in collaboration with the Washington University School of Medicine at St Louis (WashU), are to be conducted on 175 healthy volunteers aged between 18-60 years at four sites – Hyderabad, Nagpur, Patna and Chennai.
Post the screening of volunteers, the administration of the vaccine doses has begun, said sources at Hyderabad’s St Theresa’s Hospital, one of the trial sites.
“We began screening on Monday and started dosing volunteers from Wednesday. We have already administered the vaccine to about 10 volunteers,” a source at the hospital said.
Apart from the Hyderabad hospital, the Gillurkar Multispecialty hospital at Nagpur, All India Institute of Medical Sciences (AIIMS) at Patna and the Apollo Specialty Hospital at Chennai will be conducting the vaccine trials, as per the CTRI (Clinical Trials Registry-India) website.
While the institutional ethics committees of the Hyderabad, Nagpur and Patna hospitals have already approved the trials, the Chennai hospital is still awaiting the ethics committee nod.
As part of the randomized, double-blinded trial to evaluate the reactogenicity, safety and immunogenicity of the vaccine candidate, the volunteers will be divided into three groups.
In the first group, 70 volunteers will be administered a single dose of the vaccine followed by a placebo after 28 days.
In the second group 70 volunteers will be given two doses of the vaccine 28 days apart and in the third group 35 volunteers will get two doses of the placebo (a substance that has no therapeutic value).
The company will conduct an interim analysis on day 42 after the administration of the first dose to ascertain the vaccine’s immunogenicity and safety before submitting the data to the CDSCO (Central Drugs Standard Control Organization).
Bharat Biotech plans to produce 1 billion doses of the non-invasive, vaccine, which is being touted as a game-changer as it will not only be quicker but also easier to administer.
